EU and U.S. revisit PFAS regulations, federal damage awards trend higher, MAHA Commission releases childhood health strategy, and more
Monthly newsletter Material Concerns: Legal Updates on Substances of Emerging Concern keeps clients informed on the latest legal, regulatory and scientific developments related to substances of emerging concern. Each issue delivers concise, actionable insights to help companies navigate the evolving landscape of environmental law.
EPA Asks Court to Partially Vacate Rule Regulating PFAS in Drinking Water
On September 11, 2025, the U.S. Environmental Protection Agency (EPA) asked the United States Court of Appeals for the D.C. Circuit in American Water Works Ass’n v. EPA, Case No. 24-1188 (D.C. Cir.), to partially vacate its 2024 Rule regulating six per- and polyfluoroalkyl substances (PFAS) as drinking water contaminants. Although EPA initially defended the rule, the agency now asserts that portions of the rulemaking process violated the Safe Drinking Water Act (SDWA), rendering those parts of the rule invalid. Specifically, EPA requested that the court vacate its decisions to regulate three PFAS individually—PFNA, PFHxS, and HFPO-DA—and to regulate those three PFAS, along with PFBS, as mixtures through a hazard index (collectively, the Index PFAS). EPA also seeks to vacate the associated Maximum Contaminant Level Goals (MCLGs) and Maximum Contaminant Levels (MCLs). Notably, EPA is not asking the court to vacate the portions of the Rule related to PFOA and PFOS.
EPA’s motion for vacatur presents a question of statutory interpretation. The agency contends that its process for setting MCLs for the Index PFAS was unlawful because it proposed MCGLs and MCLs for the Index PFAS concurrently with its preliminary decision to regulate the Index PFAS. According to EPA, this procedural shortcut violated Section 1412 of the Safe Drinking Water Act, 42 U.S.C. § 300g-1, by bypassing a statutorily required notice-and-comment period.
But EPA’s new position does not necessarily control the outcome of the litigation. Several groups have intervened and will respond to EPA’s motion by September 26, 2025. And in the wake of Loper Bright Enterprises v. Raimondo, 603 U.S. 369 (2024), it is unclear how much weight the court will afford EPA’s interpretation of Section 1412. Further, as EPA recognizes in its brief, the court must also consider the seriousness of the agency’s error and the disruptive consequences of an interim change in deciding whether to vacate the Rule. See Allied–Signal, Inc. v. Nuclear Regulatory Commission, 988 F.2d 146, 150–51 (D.C. Cir. 1993). It is unclear how the court may balance these considerations in the event it determines EPA’s rulemaking process for the Index PFAS was unlawful.
That said, as noted in the June 2025 issue of Material Concerns, EPA previously announced plans to withdraw federal regulations concerning the Index PFAS. Accordingly, even if the court denies EPA’s motion, the agency may pursue alternative avenues—such as new rulemaking—to eliminate these standards. As a result, uncertainty surrounding the regulation of the Index PFAS is likely to persist for the foreseeable future.
As mentioned, EPA is not seeking to vacate MCLs for PFOA and PFOS. Relatedly, on September 17, 2025, in Chamber of Commerce v. EPA, Case No. 24-1193 (D.C. Cir.), EPA stated that it will defend a 2024 Rule designating PFOA and PFOS as hazardous substances under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). In a news release that same day, EPA Administrator Lee Zeldin reaffirmed the agency’s commitment to regulating PFOA and PFOS. Zeldin went further in calling for “new statutory language from Congress to fully address [EPA’s] concerns with passive receiver liability.” The news release also stated that EPA will adopt a “Framework Rule” to provide a “uniform approach to guide future hazardous substance designations.” Accordingly, manufacturers, users, and dischargers of PFOA and PFOS should expect ongoing compliance obligations under the SDWA and CERCLA. Moreover, if Congress acts on EPA’s request, these entities may face heightened liability risks under an updated statutory framework.
IARC to Evaluate Several Plasticizers Used in Consumer Products
The International Agency for Research on Cancer (IARC) will convene a Working Group to evaluate the carcinogenic potential of three plasticizers: tris(chloropropyl) phosphate (TCPP), butyraldehyde, and cumyl hydroperoxide. This evaluation is part of the IARC Monographs Volume 141, which will be held from March 3 to March 10, 2026. TCPP is used as a flame retardant in articles such as textiles and polyurethane foams used in furniture, and is also used in construction products, electronics, paint, coating and adhesives. Butanal is used in accelerators, synthetic resins, solvents and even in food as a flavoring agent. Cumyl hydroperoxide is an important oxidizer and used in the polymerization process. Industries that use and rely on these substances should be on the lookout for IARC’s conclusions in the middle of next year. Because of the manner in which agents are identified for evaluation, it is not uncommon for IARC to classify chemicals to be least “possibly carcinogenic.” Industry should prepare for potential formulation requirements, work safety standards, risk assessments and litigation.
Federal Damage Awards Are Trending Higher
Damage awards in federal court cases have climbed to unprecedented levels. According to Lex Machina’s Damage Awards Litigation Report 2025, each of the past two saw average damage awards higher than any other year on record.
The report analyzes data from over 73,000 federal cases with finalized damages awards between 2015 and 2024. It offers an overview of trends across civil litigation, as well as by judgment source and practice area.
The most dramatic growth has occurred in jury verdicts. In 2024, the average jury award exceeded $16 million, nearly double the 2022 average, which itself had doubled since 2019. The median jury award also showed significant growth, reaching $680,000 in 2024. This marks the first time median jury awards have surpassed $500,000.
In contrast, damages awarded by non-jury sources, such as judges, have fluctuated over time, showing no consistent trend.
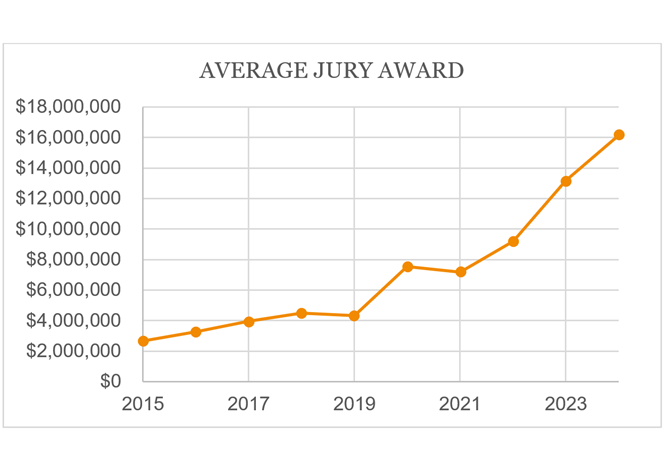
See Lex Machina, Damage Awards Litigation Report 2025 (providing data).
Tort claims have also seen a significant increase in damage awards. Between 2020 and 2024, the average award climbed 2.5 times higher than between 2015 and 2019. Median awards grew 1.5 times higher, indicating that typical damages have increased and the largest verdicts have surged even more. Average damages awards in environmental claims brought under federal statutes, class actions and consumer protection cases did not increase during this period but median awards did see substantial growth. This pattern suggests that extreme awards declined, while the bulk of cases shifted upward. Specifically, median awards rose 32% in environmental cases and over 50% in class actions and consumer protection cases.
While the report does not offer a definitive explanation for these trends, it raises several possible contributing factors. These include expanded litigation funding, shifting social attitudes about who should absorb risk, and a tactic by plaintiffs’ attorneys who urge jurors to reach verdicts that “send a message” to defendants.
Senator Proposes CERCLA PFAS Liability Protections in 2026 Defense Bill
On July 31, Sen. Pete Ricketts (R-NE) proposed an amendment (S.A. 3363) to the Fiscal Year 2026 National Defense Authorization Act (NDAA) that would significantly alter how liability is assigned under Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) for certain entities involved with per- and polyfluoroalkyl substances (PFAS). Under CERCLA, liability for the release of hazardous substances is assigned to “potentially responsible parties” (PRPs) who are: 1) the current owner or operator; 2) the owner or operator of a facility at the time of disposal; 3) a person who arranged for the disposal or treatment of hazardous substances; or 4) a person who transported hazardous substances and selected the site for disposal. Parties are held strictly, jointly and severally liable for contamination and the cost of the cleanup. The amendment would exempt “fire suppression entities,” or entities that use a fire suppression system, water and wastewater utilities, agricultural producers, and resource management facilities from cleanup costs related to the release of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS)—provided their activities were conducted in compliance with applicable laws and standards. These exemptions aim to protect “passive receivers” of PFAS, or entities that receive PFAS but did not produce them, from financial responsibility for environmental cleanups.
In addition to liability protections, the amendment proposes a narrower definition of PFAS than what lawmakers previously enacted in NDAA legislation in Fiscal Years 2021, 2022 and 2023 and what 23 states have also adopted. Currently, PFAS is defined as chemicals with only one fluorinated atom. Ricketts wants to limit it to substances with “at least 2 fully fluorinated saturated carbon atoms” and is either “a non-polymeric substance” or a “side-chain fluorinated polymer.” The amendment also defines “fully fluorinated saturated carbon atom” to include “a perfluorinated methyl group (–CF3) or a perfluorinatedmethylene group (–CF2–).” Furthermore, PFAS would include “any degradant” that meets the revised definition but exclude “any compound in which the fluorinated carbon atoms are unsaturated.” This redefinition could reduce the number of chemicals subject to regulation and liability. If adopted, the amendment could shift the regulatory landscape by narrowing the scope of PFAS oversight and shielding certain downstream users from costly litigation.
Local Cape Fear River Communities Request State Regulation of 1,4-Dioxane
In a series of letters to the North Carolina Environmental Management Commission (EMC) and the North Carolina Department of Health and Human Services (DHHS), downstream communities and advocates for Cape Fear River, North Carolina, are asking the state to regulate 1,4-dioxane in the state’s waterways.
Background on 1,4-Dioxane
1,4-dioxane is used as a solvent in the manufacture of a variety of products such as adhesives, sealants, spray polyurethane foam, printing inks and paint strippers. 1,4-dioxane is also a byproduct in the manufacture of polyethylene terephthalate and has been found as a contaminant in soaps and detergents. The U.S. Environmental Protection Agency (EPA) classifies 1,4-dioxane as a “likely human carcinogen.”
Currently, there are no federal regulations for 1,4-dioxane in drinking water. But EPA recommends drinking water levels of 0.35 parts per billion (ppb), which it claims represents a one in a million cancer risk level over a lifetime exposure.
1,4-dioxane can be removed from water using ozone and biological filters. Using these processes, the water is injected with ozone to chemically break down contaminants before passing through special filters containing microbes that eat the remaining contaminants.
1,4-Dioxane Found in Cape Fear River Basin
The state of North Carolina asserts that it has the nation’s third-highest concentration of 1,4-dioxane and that within the state, the Cape Fear River Basin has some of the highest levels of 1,4-dioxane in the country.
The Cape Fear River Basin is the largest river basin in the state. It runs through New Hanover County where it drains into the Atlantic Ocean. 1,4-dioxane was first detected in the Cape Fear River Basin as early as 2014. Water samples taken as recently as July 2025 showed 1,4-dioxane still present at levels of 0.55 ppb in raw water, and at 0.09 ppb in treated water, with multiple upstream sources identified by the state as contributing to the contamination.
Local Communities Request Regulation
Attempts by the state to regulate 1,4-dioxane in drinking water have been unsuccessful. In September 2024, an administrative law judge found that the North Carolina Department of Environmental Quality (DEQ) exceeded its authority when it sought to regulate 1,4-dioxane in wastewater discharge because EPA classified the chemical only as a Group B, “probable” human carcinogen and not as a more definitive Group A, human carcinogen.
In November 2024, the Cape Fear Public Utility Authority (CFPUA) unsuccessfully petitioned EMC, the rulemaking authority for DEQ’s Divisions of Water Resources, for an emergency rule that would require 1,4-dioxane dischargers to submit a minimization plan to reduce their 1,4-dioxane discharges by 80% over five years. CFPUA’s petition was denied because it did not meet the legal requirements for an emergency rule.
This year, the local communities of Cape Fear River continue their efforts to seek state regulation of 1,4-dioxane. In March 2025, the New Hanover County commissioners requested the EMC set clear, enforceable limits on the discharge of 1,4-dioxane.
In July and August, the CFPUA, Wilmington City Council, and the Southern Environmental Law Center—on behalf of Cape Fear River Watch, Haw River Assembly, NC Conservation Network, North Carolina Sierra Club, and Toxic Free NC—each separately requested DHHS to set a preliminary health goal for 1,4-dioxane in drinking water. A preliminary health goal is a non-enforceable target for contaminants in drinking water and a first step towards eventual regulation. The preliminary health goal is based on the level at which a contaminant is not expected to cause health effects in people. It does not take into account the practical cost or technical feasibility of reducing contaminants to those levels through existing wastewater treatment options.
In response to these multiple letters, DHHS stated that it is working with DEQ to address the issue. EMC, however, has not yet taken any steps to start the rulemaking process to limit 1,4-dioxane in the water.
EPA’s Final Rule Adding Additional PFAS to the Toxic Release Inventory: Changes Possible, Further Delay Likely
In October 2024, during fiscal year 2025, the Biden EPA proposed a rule to add 16 individual PFAS and 15 PFAS categories to the Toxic Release Inventory (TRI) and to designate them as chemicals of special concern (CSC). All told, under the proposed rule, more than 100 individual chemicals would join the more than 200 PFAS already on the TRI. In addition, the rule would set a 100-pound reporting threshold requiring facilities to count all PFAS in a given category cumulatively. However, nearly a year and a new presidential administration later, EPA has yet to finalize the proposed rule.
Despite the delay, President Donald Trump’s recently released proposed EPA 2026 fiscal year budget reflects its intent to finalize the Biden-era proposed rule by setting aside an additional $1.2 million for the TRI program to address additional PFAS listings. However, the agency also signaled that changes and further delay to the proposed rule’s implementation are also likely, stating “EPA anticipates finalizing the proposal in a manner consistent with the [Trump] Administration’s priorities and policies in FY 2026 or FY 2027.”
It is unclear how the Trump EPA may change the proposed rule, it appears likely the agency will remove the CSC designation, allowing facilities with TRI obligations to utilize the de minimis exemption that allows facilities to disregard concentrations of chemicals in mixtures or trade name products under one percent when reporting releases and making other waste management calculations.
Although both the exact timeline for implementation and the final form the of the Biden-era proposed rule remain uncertain, the Trump EPA appears committed to the rule for now. In turn, clients that utilize PFAS for manufacturing, processing or otherwise should remain cognizant of the rule’s potential implications.
Make America Healthy Again Commission Releases Childhood Health Strategy
On September 9, 2025, the Make America Healthy Again (MAHA) Commission—established by President Donald Trump through executive order—released its long-awaited “Make Our Children Healthy Again Strategy” (Strategy Report). This report is a follow up to the Commission’s “Make Our Children Healthy Again Assessment” (MAHA Report), published on May 22, 2025 (check out our analysis in the June 2025 issue of Material Concerns). The Strategy Report claims to outline “a strategic approach for executive actions to address the childhood chronic disease crisis” through advancing data collection and research, realigning incentives, increasing public awareness and knowledge, and fostering private sector collaborations. Though the report includes recommendations related to childhood nutrition, mental health and vaccines, we will focus on directives and recommendations related to environmental chemicals like persistent organic pollutants and pesticides, PFAS, microplastics, phthalates, bisphenols and fluoride, calling for additional and more coordinated research. For example, it directs the Department of Health and Human Services to evaluate the risks and exposures of microplastics and synthetics in common products like textiles. Specifically, the report directs the Environmental Protection Agency (EPA), the U.S. Department of Agriculture (USDA), and the National Institutes of Health to collaboratively research and evaluate cumulative exposure to environmental chemicals, placing a specific emphasis on leveraging new approach methodologies. It also directs EPA to focus research on pesticides that act through a common mode of action. The report also directs EPA and USDA to collaborate with other federal agencies to continue to evaluate water contaminants and to update guidance when appropriate.
While the Strategy Report emphasizes research and innovation, it does include proposed targeted regulatory initiatives aimed at endocrine disruption affecting infants and children allegedly caused by phthalates and bisphenols. The report further directs the Food and Drug Administration (FDA) to implement policies to limit or prohibit the use of petroleum-based food dyes and close the “Generally Recognized as Safe” loophole. And the report directs FDA to develop and implement an “enhanced evidence-based systematic process for the post-market assessment of chemicals in food,” including those that are unintentionally present like environmental contaminants. The report goes on to instruct the Centers for Disease Control and Prevention to update recommendations for fluoride and PFAS in water.
Shifting gears slightly, the report also calls for regulatory streamlining by directing EPA to “reform the approval process for the full range of chemical and biologic products to protect against weeds, pests, and disease to increase the timely availability.” In furtherance of its goal to foster inter-agency pesticide research initiatives, the Strategy Report directs EPA to partner with industry stakeholders to ensure the public is aware of and confident in EPA’s pesticide review procedures and their capacity to reduce risks for pesticide users and the general public. The report also instructs USDA and EPA to partner with the private sector to improve soil health and reduce pesticide load by investing in precision agriculture technologies.
For companies that manufacture or use chemicals, the initiatives outlined in the Strategy Report signal the potential for increased scrutiny from the government and the general public, as well as evolving expectations for transparency and safety.
Agricultural Industry Pushback Mounts Against EPA’s PFAS Biosolids Risk Assessment
The Environmental Protection Agency’s (EPA) January 2025 draft risk assessment on PFAS in biosolids has sparked concern across the agricultural industry. The draft risk assessment is intended to guide future regulations from federal and state agencies regarding PFAS and biosolids, while ensuring the agricultural industry continues as a vital infrastructure for the American economy. However, both state regulators and the agricultural industry argue that the models used within the draft risk assessment rely on extreme assumptions that do not account for realities in modern agriculture. Critics warn that the proposed 1 ppb threshold could effectively ban biosolids use, eliminating a cost-effective fertilizer and increasing operational burdens for farmers and municipalities alike, in a landscape of already high agricultural farming input costs.
Among the criticisms leveled by these critics is EPA’s use of a “farm family” model based on decades of hypothesized biosolids application and antiquated notion that farm families exclusively consume farm-grown food and locally obtained water. The National Milk Producers Federation, which represents the dairy industry, commented it is “aware of dairy farms that have supplemented their land with biosolids, but we cannot imagine any farm that would or has done that every year for 40 years.”
EPA’s data and analysis are also subject of industry comments. In their comment letter, the Illinois Farm Bureau, the Illinois Corn Growers Association and the Illinois Soybean Growers criticized EPA’s reliance on “extreme assumptions” regarding modeling data. The Maine Organic Farmers and Gardeners Association specifically criticized EPA in its comment for excluding Maine’s current research on the topic. Maine was first state to ban the land application of biosolids. Others indicate that EPA did not consider existing state regulations, such as the comment from the Virginia Department of Environmental Quality.
Although it is still unknown how the current administration will address these concerns, if at all, EPA has signaled potential intention to revisit the science behind the draft risk assessment. While the draft risk assessment does not have the power of a final regulatory action, it is likely to inform the conversation around the future regulation of PFAS and biosolids on both a state and federal level. For agribusinesses operating in environmental consulting, waste management or agricultural services, this evolving regulatory landscape presents both challenges and opportunities in navigating PFAS compliance and biosolids reuse.
EU Proposes to Curtail Sweeping PFAS Ban, Exempt Some Fluoropolymers
In a major shift from its original plan, the European Union (EU), acting through the European Chemicals Agency (ECHA), has proposed a scale-back of its sweeping 2023 proposal that would have phased out the manufacture, import and use of per- and polyfluoroalkyl substances (PFAS) within the EU. The initial proposal, submitted on January 12, 2023, aimed to impose one of the most comprehensive bans on PFAS globally, including either a full ban on PFAS, or a ban with some very limited use-specific exceptions. A six-month public comment period generated more than 5,600 submissions, prompting regulators to reconsider its one-size-fits-all approach.
On August 20, 2025, based on its review of scientific and technical evidence, the EU members submitted a revised proposal allowing for additional use-specific exceptions in addition to conditional exemptions for certain fluoropolymers—materials considered critical for sectors including medical devices, energy and electronics. The revised proposal recognizes the unique properties of fluoropolymers, which are difficult to replace without compromising safety and performance in essential technologies. The revision also proposes regulatory options beyond a blanket ban, such as controlled use under strict emission-reduction measures.
The ECHA’s Risk Assessment and Socio-Economic Analysis Committees are currently reviewing the updated proposal and will continue technical discussions through late 2025. The ECHA will then issue its final opinion, at which point the European Commission will draft the legislation text. The proposal will then go through each EU member state’s vote and require European Parliament approval, likely sometime in early 2027.
EU Bans TPO in Cosmetics Under New CMR Regulation
Effective September 1, 2025, the European Union will now prohibit the use of Trimethylbenzoyl Diphenylphosphine Oxide (TPO) in all cosmetic products, including professional-use items such as UV-cured nail gels and hybrid polishes. This regulatory shift stems from the 7th CMR Omnibus Regulation, which adds 22 substances to Annex II of the EU Cosmetics Regulation—reserved for ingredients classified as carcinogenic, mutagenic or toxic to reproduction (CMR). TPO, previously permitted for professional use at concentrations up to 5%, has now been reclassified as a Category 1B CMR under the EU’s Classification, Labelling and Packaging (CLP) Regulation, increased from a Category 2 CMR designation in 2012. This classification indicates sufficient evidence of reproductive toxicity, prompting its full prohibition in cosmetics.
The European Commission has emphasized that there will be no transitional period. As of the effective date, products containing TPO must be withdrawn from the market, and their continued sale or use will be non-compliant. Distributors are instructed to cease supply, and salon owners must verify ingredient lists, discontinue use, and dispose of affected products. This regulatory update follows a multi-year review process, including a 2020 safety reassessment initiated by Swedish authorities and a formal reclassification in 2023. Legal and compliance teams in the cosmetics and personal care sectors should ensure their product lines and supply chains are aligned with the updated Annex II listings. Manufacturers and importers are also advised to monitor the Scientific Committee on Consumer Safety (SCCS) for additional substances under review, as further updates to the Cosmetics Regulation are anticipated. The commission has published a Q&A resource to assist stakeholders in navigating the implications of the TPO ban.
This development underscores the EU’s continued commitment to consumer safety and the precautionary principle in cosmetic regulation. Legal counsel should advise clients on proactive compliance strategies and potential liability risks associated with non-conforming inventory post-September 1.
EPA’s Proposed Rule Could Ease TSCA PFAS Reporting—Now Under Review
On August 29, 2025, the Environmental Protection Agency (EPA) submitted the proposed rule titled “Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) Data Reporting and Recordkeeping under the Toxic Substances Control Act (TSCA); Revision to Regulation” to the Office of Information and Regulatory Affairs (OIRA) for review. Although the text of the proposed rule is not yet available, EPA’s proposed rule is expected to incorporate “certain exemptions and other modifications to the scope of the reporting rule.” Currently, the import of municipal solid waste streams for disposal or destruction of waste is the only exemption for non-federal agencies in the PFAS Reporting Rule. In October 2023, EPA estimated the cost of PFAS reporting and recordkeeping requirements to be $800 million to $843 million. The high cost imposed on those subject to the rule’s reporting requirements has led to much criticism. The proposed rule could significantly ease TSCA PFAS reporting requirements and its associated costs.
Pursuant to the TSCA PFAS Reporting Rule, 40 C.F.R. Part 705, manufacturers of PFAS or PFAS containing products and companies importing products made with PFAS during the 11-year period between January 1, 2011, and December 31, 2022, are required to report certain data to EPA through the Central Data Exchange regarding PFAS uses, manufactured amounts, exposures, environmental and health effects. The rule’s reporting requirement has been delayed multiple times at least in part due to industry’s need to beta test the Central Data Exchange. Most recently, EPA’s May 2025 interim final rule extended the dates for submitting the required data under the PFAS Reporting Rule to April 13, 2026, through October 13, 2026, for most manufacturers and importers and giving small manufacturers reporting exclusively as article importers until April 13, 2027.
Although the details of EPA’s proposed rule will not be known until it is published in the Federal Registrar, the EPA has suggested that the proposed rule could ease reporting requirements for small manufacturers and article importers. EPA Administrator Lee Zeldin outlined the agency’s action plan for PFAS which includes “implement[ing] TSCA section 8(a)7 to smartly collect necessary information, as Congress envisioned and consistent with TSCA, without overburdening small businesses and article importers.”
According to the RIN Data on OIRA’s website, the projected date for publication of the proposed PFAS Reporting Rule is December 2025. The projected date for the final rule is June 2026, which falls between the current submission period for most manufacturers. Therefore, EPA could again extend the submission of the reporting requirements until after the effective date of the final rule.











